FDA APPROVED - Cleared 510 K
Sapheneia was the first third-party low dose FDA approved Software Company in the United States. Sapheneia-Scannerside was the first third party company to combine low dose with dose monitoring in the United States. At RSNA 2014, Sapheneia-Scannerside became the first Single Source Solution which included XR-29 Dose Check Upgrades. To date, not all XR-29 Solution Companies can provide Dose Reduction and Custom Protocols once the XR-29 Monitoring indicates your dose levels are too high. To date, no other third party company has been around as long as Sapheneia and no other company has as many systems successfully operating worldwide.

RSNA 2014-Single Source Solution with XR-29 Dose Check Introduction
Scannerside™ XR-29 Dose Check was developed for legacy CT Scanners that do not have the MITA XR-29 requirement for Alerts. This solution is custom designed for your specific scanner operating system. XR-29 Dose Check was designed not to be invasive to the manufacturer's equipment or operating firmware. Not only is this solution non-invasive, our architecture includes an extra medical monitor at the console so that in the future it is more cost effective for a facility to install low dose software as the XR-29 monitoring identifies protocols with higher than desired dose levels.
For any specific questions regarding this solution please contact Sapheneia-Scannerside at sales-usa@sapheneia.com 1-888-342-5619
Key Features
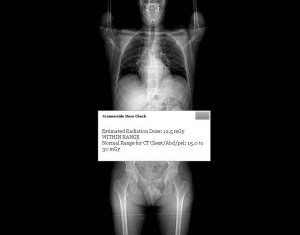
- Pre-scan patient specific radiation dose estimate.
- Pre-scan warning of high radiation dose.
- Required acknowledgment of dose estimate by technologist.
- Technologist data entry box for explanation of high potential radiation dose.
- Full Compliance - Letter of Compliance / Certificate of Compliance with FDA Clearance
- Frequently Asked Questions for XR-29
- Professional Industry Links
- CMS Response to MITA - Regarding Certifications of 3rd Party Solutions
- XR-29 Dose Check Specifications
- ACR XR-29 FAQ